Preventing concrete degradation in sulfate-rich environments
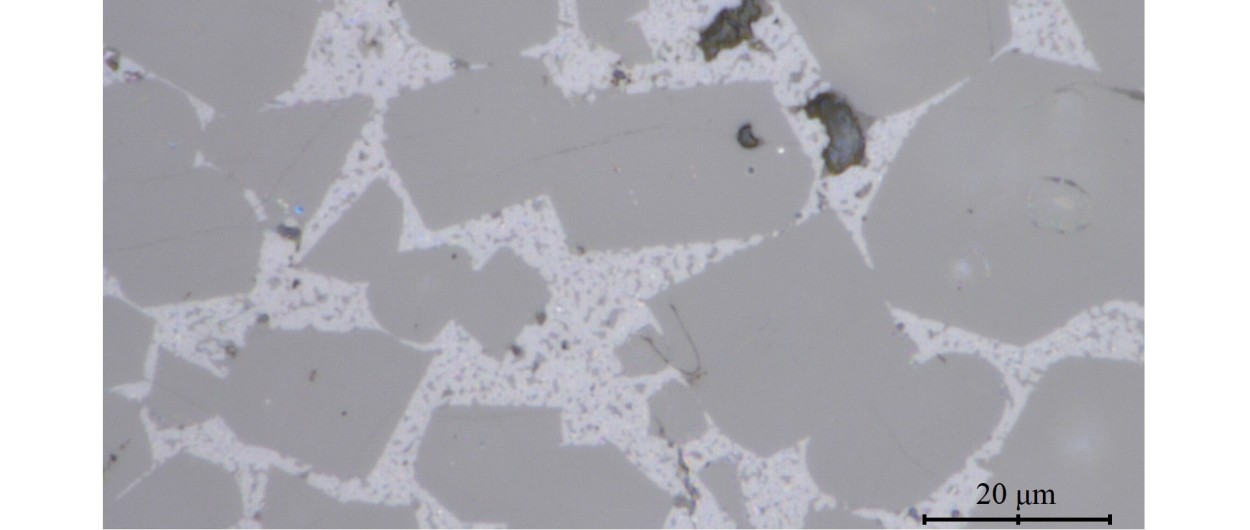 Optical microscope image of cement adapted to sulfated environments. Ferrite crystals are the smallest (brightest contrast).
Optical microscope image of cement adapted to sulfated environments. Ferrite crystals are the smallest (brightest contrast).
Concrete, which has been manufactured since antiquity, is one of the most widely used construction materials. But it has weaknesses. Near the sea or in areas where the soil is rich in gypsum, calcium sulfate can seep through the pores of the concrete, causing a reaction that makes the material swell and then crack, with serious consequences. In France, this type of gypsum-rich soil is found, for example, throughout much of the Paris Basin and in the Alps. To prevent these accidents, concrete designed to be resistant to calcium sulfate attacks has been developed using special cements. However, even these materials can be vulnerable. The LSI is one of the laboratories called upon by manufacturers to help understand the origin of the problem.
Concrete is based on cement. When mixed with water, cement forms a paste that binds sand and gravel grains together. But cement is a more complex material than it appears. It mainly contains four minerals: two calcium silicates and two calcium aluminates, which are tricalcium aluminate and calcium ferrite. “The composition of these mineral phases depends on the raw materials and manufacturing conditions, such as the fuels used to power the kiln,” explains Mireille Courtial, a researcher at LSI. In this study, the scientists were able to work with cement manufactured directly by industrial producers.
Selective chemical dissolution
To mitigate the risk associated with sulfates, current cements limit the presence of tricalcium aluminate. Given the persistence of the problem, researchers have turned their attention to the role of calcium ferrite, which had been little studied until now. The first challenge was to isolate it. "Micrometric ferrite crystals are entangled with other phases. The usual techniques are not sufficient," explains Marie-Noëlle de Noirfontaine, also a researcher at LSI. As part of Alexis Mériot's thesis, LSI scientists developed a two-step selective attack method to dissolve unwanted minerals.
Next, a systematic study of calcium ferrites was conducted. Thanks to the DIFFRAX platform at École Polytechnique, which has state-of-the-art X-ray diffractometers, the crystalline structure of calcium ferrites could be studied in greater depth. This information was compared with data obtained using other methods, such as electron probe microanalysis, which is capable of counting the atoms of each species.
The role of calcium ferrites
“Our analyses enabled us to differentiate between very similar ferrite compositions in four industrial sulfate-resisting cements. We identified two families of ferrites, which cause a difference in reactivity with calcium sulfates" explains Mireille Courtial. This reactivity was then studied in detail at the Laboratoire interdisciplinaire Carnot de Bourgogne and published in a second article.
The reaction of concrete to calcium sulfates depends on several parameters of the construction environment, and this research shows that calcium ferrites can no longer be ignored. “The X-ray diffraction methods we used can be applied by cement manufacturers to control their processes. This could lead to a change in cement standards,” explains Marie-Noëlle de Noirfontaine. “This is an example of how fundamental research directly helps industry.”
*LSI: a joint research unit CEA, CNRS, École Polytechnique, Institut Polytechnique de Paris, 91120 Palaiseau, France
 Support l'X
Support l'X